Core Insights - The REPO4EU platform
Welcome to REPO4EU: Core Insights
Our brand new article series where we bring you closer to our project's core innovations and results.
The REPO4EU platform
A brief introduction
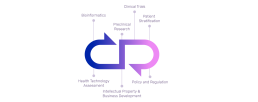
At the heart of REPO4EU lies a bold ambition: to build a cutting-edge, modular, and user-friendly digital platform that empowers researchers, clinicians, and regulators to collaboratively drive drug repurposing efforts in a structured and scalable way. Our goal is to provide the technical backbone for the entire REPO4EU ecosystem —a place where tools, data, methodologies, and collaborations converge to streamline and accelerate repurposing workflows across Europe.
The platform will not only integrate existing computational tools and data resources, but also serve as a dynamic workspace where users can conduct complex in-silico analyses, access curated scientific databases, and examine comprehensive research documentation to ensure transparency and reproducibility. An integrated matchmaking system enhances this environment by fostering interdisciplinary collaboration and connecting users with relevant expertise, tools, and data.
Built on principles of transparency, FAIR data, and cross-disciplinary usability, the REPO4EU platform aims to redefine how repurposing research is conducted and transformed into actionable insights.
The road so far
A quick walk through the development journey
The requirement-gathering phase for the platform was successfully completed last summer, providing us with a clear and validated understanding of user needs. These initial insights have shaped the ongoing technical implementation and ensured the platform is built around actual use cases.
The focus so far has been on the development of the platform’s core components that will support long-term functionality and scalability. This includes the implementation of a robust backend architecture, a flexible Content Management System (CMS), an interactive decision tree engine component to support guided user workflows, a curated database module for structured resource management, and the foundation for a platform assistant to support user navigation.
The most immediate challenge now is ensuring the platform can adapt to the wide variety of tools, datasets, and user needs; each with different technical expectations and degrees of complexity. Unlocking compliance, scalability, and performance without compromising security, and balancing flexibility with usability is a constant priority. On top of this, rapid developments in AI and data privacy require careful technical and ethical considerations as we implement features like AI-powered assistance or advanced analytics.

Looking ahead
The driving force - Putting together the pieces of the drug repurposing puzzle
The strong interdisciplinary nature of REPO4EU remains our strongest motivation. Collaborating with experts in regulatory science, pharmacology, bioinformatics, and clinical practice has been energizing, and it keeps our focus grounded in real impact. After all, our biggest hope is to create a platform that doesn’t just serve today’s needs, but is able to grow into a sustainable digital infrastructure for drug repurposing across Europe and beyond.
The opportunity to see the platform evolve into a truly user-centric, interoperable ecosystem that enables researchers, clinicians, and decision-makers to collaborate and innovate more efficiently in the field of drug repurposing is what excites us the most moving forward. With so many diverse tools and resources being integrated, and the emergence of new technologies like AI-driven assistants, we see REPO4EU becoming a key enabler for next-generation, evidence-based medicine.
We believe that bringing complex workflows, such as in-silico modeling, sustained by a solid research collection and a navigable network of drug repurposing prior art into a unified interface that doesn’t overwhelm users but instead guides them through well-structured, meaningful paths could genuinely lower the barrier to entry for impactful research and accelerate the path from idea to implementation, especially in the drug repurposing realm.
The recent momentum in generative AI has also opened exciting new directions. To better support users navigating these repurposing workflows, we are now developing an AI Assistant Chatbot. This assistant will help users find the right tools, resources, and data based on natural language queries, significantly improving user experience, accessibility and encouraging meaningful engagement.
At the same time, we are aware of the massive challenge of stitching all these components together in a way that’s both technically robust and intuitive to use. Ensuring scalability, long-term maintainability, and data governance compliance while staying agile and user-driven is a constant balancing act. After all, we understand that successful adoption hinges not only on specific features but on clarity, performance, and trust — all factors that require continuous fine-tuning and community feedback.

Behind the scenes
Meet our development team
The platform development team, led by Egnosis, is responsible for the overall design, technical development, and iterative rollout of the platform. Their expertise lies in systems integration, data architecture, workflow design, and user-centered software engineering. They act as both developers and facilitators, and transform scientific needs into working, scalable tools.
Key contributors to this effort include experts in cloud-based infrastructure, data modelling, secure user access, and research software engineering. The development team works hand-in-hand with REPO4EU partners across the whole consortium to ensure the platform not only meets technical requirements but also aligns with the practical workflows of its users.
About our platform
Discover our platform and explore its key features
Become an early tester now!
Follow the link to sign up for our platform's alpha release
Drug Repurposing Next-Gen: Interview with Pablo Perdomo (Technical University of Madrid)
Pablo Perdomo is a PhD researcher at the Technical University of Madrid (UPM). Originally from Gran Canaria, Pablo holds a degree in Biomedical Sciences from the Autonomous University of Barcelona and a master’s in Bioinformatics from Leiden University in The Netherlands. In this interview he talks about building a career as a data scientist, and shares an insight into his current role as a researcher at UPM, where he’s exploring the intersection of graphs, artificial intelligence and bioinformatics within REPO4EU.
From Biomedical Sciences to Bioinformatics
Hi Pablo! To start off, could you tell us what first inspired you to pursue a career in science?
When I started my bachelor’s in biomedical sciences, I was initially drawn to the biology side of things. But near the end of my degree, I took a bioinformatics course and found it fascinating, and I decided to focus on this field from that point on. I took additional courses in informatics and AI, and eventually found a master’s programme at Leiden University that perfectly matched my interests. My master’s thesis in the BioSemantics group introduced me to knowledge graphs and drug repurposing — the two main areas I continue to work on today at UPM.
What was it about artificial intelligence that caught your attention?
My uncle, who’s a university professor, introduced me to some of the early breakthroughs in AI — like AlphaGo and AlphaZero. Seeing how machines could outperform humans in complex games felt almost magical. That was when I realised how powerful and fast-moving this field was, and it really motivated me to pursue AI in a scientific context.
Given your interest in AI and computer science, what made you choose bioinformatics instead of going fully into computer science?
I didn’t want to completely abandon my background in biomedical sciences. Bioinformatics felt like the perfect bridge between the two worlds — technology and biology. My mother is a doctor, so I grew up surrounded by healthcare and with a strong sense of wanting to help people. That connection to medicine is something I wanted to preserve in my research.
Exploring knowledge graphs in drug repurposing
Let’s talk a bit about your current work. What questions are you trying to answer in your research?
At UPM, I’m focusing on AI techniques applied to knowledge graphs — data structures made up of nodes and edges that represent relationships, similar to a social network. In the context of REPO4EU, we use these graphs to connect genes, diseases and symptoms, allowing us to identify new drug candidates for existing diseases.
But I’m especially interested in explainability — not just generating predictions, but also understanding why a model suggests a certain drug. Providing explanations or hypotheses helps increase trust in AI-driven discoveries.
What was your first experience with drug repurposing research?
My first real contact with drug repurposing came during my master’s thesis at Leiden, where I worked on knowledge graphs for drug repurposing in the BioSemantics group. Before that, I didn’t know much about the field. But once I started, I was amazed by its potential to make a real difference in people’s lives. It’s incredibly rewarding to know that your work could eventually help patients.
The power of collaboration in evolving research fields
One of the core ideas behind REPO4EU is interdisciplinary collaboration. How has it been for you to work with experts from different fields like medicine, bioinformatics and computer science?
I really enjoy it. My background is in biomedical sciences, so working closely with people in medicine and biology gives me a sense of connection to where I started. At the same time, I get to apply advanced computer science tools to solve real biological problems. It’s the best of both worlds, and it keeps things exciting and meaningful.
You’re working at the intersection of two rapidly evolving fields: AI and drug repurposing. What excites you most about contributing to both?
It’s incredibly motivating to know that what I’m doing could have a direct impact on improving people’s lives. I feel very lucky to work on something that I love, while also knowing it serves a real purpose. That sense of usefulness keeps me going every day.
And on the flip side — what are some of the challenges you face in your work?
The biggest challenge is definitely keeping up with how fast the field is moving. What’s new today might be outdated in just a few months. You need to constantly read, learn and adapt. It can be exhausting, but it’s also inspiring — every new development sparks new ideas and possibilities.
Advice for young scientists and future researchers
What’s the main inspiration behind your work?
My parents have had a big influence on me. My mother is a doctor, and my father is a technology teacher — so I guess I’m the perfect mix of both! That combination of science and technology is my true passion and it’s what led me to bioinformatics and AI, as I said earlier, and that’s what continues to drive my research, really.
Finally, what advice would you give to young researchers who are just starting their journey in science?
Be curious! Explore different areas until you find something you’re truly passionate about. And make sure to surround yourself with a great research group. I’ve been really lucky in that sense — both the BioSemantics group in Leiden and my current group at UPM are full of amazing people, both professionally and personally. When you feel supported and motivated in your environment, your work improves naturally.
REPO4EU: The Podcast
Our podcast brings listeners closer to the latest innovations, research and developments happening in drug repurposing across the globe. The first season, ‘Drug Repurposing Next-Gen’, spotlights the work of PhD researchers, post-docs and young investigators involved in REPO4EU, exploring their role in the project as well as their career journeys. New episodes will be released monthly. Stay tuned for the next one!
Drug Repurposing Next-Gen: Interview with Cristian Nogales (University of Vienna)
Cristian Nogales is a postdoctoral researcher at Ludwig Boltzmann Institute for Network Medicine, at University of Vienna. After pursuing studies in biochemistry and biotechnology in Spain, he completed his PhD at Maastricht University, where he eventually converged his research with the fields of systems biology, bioinformatics and drug repurposing. In this interview, he tells us about his academic journey so far and his current involvement in the REPO4EU project, where he’s investigating diagnostic and drug repurposing opportunities in the field of cardiovascular-metabolic diseases and low complexity cancers.
Science as a means to create meaningful impact
Hi Cristian! What first drew you to pursue a career in science?
I’ve always had a passion for science, even from an early age. During high school I wanted to study medicine, but at some point, I realised that it wasn’t quite the right fit for me. I still wanted to stay connected to the human and medical side of biology, though — that’s how I ended up studying biochemistry at University of Seville.
After completing my degree, I decided to continue exploring applied science and moved to Madrid for a master’s in biotechnology. I liked the idea of using science to create something tangible that could eventually help people, which set me on the path I’m still following today.
You later pursued a PhD at Maastricht University. What made you choose the academic path?
It’s actually funny because my first thought was that I didn’t want to go into academia! I wasn’t one of those people who always dreamed of doing a PhD, but an opportunity came up at Maastricht University and I decided to take it to see how I felt about research.
I quickly realised that I really enjoyed the freedom and creativity that come with academia. It allows you to explore ideas, collaborate with people from different fields and build something meaningful. So even though it wasn’t my initial plan, I’m very happy with where it led me.
What was the focus of your research for your doctorate?
I joined Maastricht University just as they were starting REPO-TRIAL, the project that later laid the foundation for REPO4EU. My research focused on cardiovascular diseases (mainly stroke and heart failure) and how to identify the molecular mechanisms that cause them.
By understanding these mechanisms, we can develop new ways to diagnose and treat patients. Over time, I became particularly interested in diagnostics, figuring out how to distinguish between patients who may have the same symptoms but different underlying biological causes. That’s key to delivering more personalised treatments.
The power behind drug repurposing
Did you know much about drug repurposing before joining these projects?
I had heard about drug repurposing during my studies in Madrid, but I didn’t fully appreciate its potential at the time. Back then, I associated it mainly with business strategies — for example, companies extending patents — rather than with its clinical and humanitarian benefits.
But once I started working on REPO-TRIAL, I understood its real value. Drug repurposing allows treatments to reach patients much faster because the drugs have already been approved for safety. If we can match an existing drug to a new disease mechanism, we can skip years of testing and immediately start improving people’s lives. That’s incredibly powerful.
You have a background in both biology and computational work. How does that shape your contribution to REPO4EU’s mission?
I think my hybrid background really helps. I come from a biological and biotechnological foundation, but I’ve always been drawn to the computational side. During both my bachelor’s and master’s theses, I worked in bioinformatics-related projects, learning to use computational tools to interpret biological data.
Now, in REPO4EU, I see myself as a bridge between two worlds — the biologists and clinicians on one side, and the bioinformaticians and data scientists on the other. These groups sometimes speak different “languages,” and I enjoy helping them understand each other’s perspectives and needs. That’s essential in a project as interdisciplinary as this one.
My work now focuses mainly on the computational aspects of the project, particularly helping to develop the REPO4EU Platform together with my colleague Chloe Bucheron. I also support the team when biological questions arise, drawing from my experience to connect the data side with the biological context.
Advice for young scientists and future researchers
They say the life of a researcher often comes with challenges. What keeps you motivated?
For me, it’s about seeing the real-world impact of what we do. Research can be difficult and full of obstacles, but when you know your work could eventually help patients, it becomes worth it.
That sense of purpose, combined with the fact that I genuinely enjoy what I do, keeps me going. It doesn’t feel like a struggle when you love the process and know it’s contributing to something meaningful.
From what you’ve experienced in your journey so far, what advice would you give to someone thinking of pursuing a PhD or a career in science?
I’d say: take it easy. Academia is fascinating, but it can also be overwhelming at times. It’s important not to put too much pressure on yourself. Go step by step, learn as you go, and try to enjoy the process.
There will be challenges, of course, but if you stay curious and passionate about your work, it’s an incredibly rewarding path. So, basically, enjoy the ride!
REPO4EU: The Podcast
Our podcast brings listeners closer to the latest innovations, research and developments happening in drug repurposing across the globe. The first season, ‘Drug Repurposing Next-Gen’, spotlights the work of PhD researchers, post-docs and young investigators involved in REPO4EU, exploring their role in the project as well as their career journeys. New episodes will be released monthly. Stay tuned for the next one!
REPO4EU researchers launch SMART, the tool that simplifies decision models for healthcare innovation
Decision-analytic models play a vital role in healthcare, helping researchers, policymakers, and clinicians evaluate the cost-effectiveness and value of new interventions. Yet, building these models can be complex, especially in early-stage research or data-limited contexts such as drug repurposing or rare diseases.
To address this challenge, researchers in the REPO4EU consortium have developed SMART, a structured, step-by-step tool designed to guide users through making, reporting, and justifying simplified modelling choices tailored to specific decision contexts.
Teebah Abu-Zahra, Health Economics Researcher at Maastricht UMC+, led the development of the tool alongside other colleagues involved in REPO4EU. In this interview, she explains the motivation behind SMART, how it can support innovators and decision-makers, and what role it plays in advancing drug repurposing and precision medicine.
Hi Teebah! Can you tell us, in a few words, what is SMART?
SMART is a practical tool built to guide researchers through the process of making, reporting, and justifying simplified healthcare modelling choices, in a step-by-step structured framework. It helps users understand the trade-offs between the model’s simplicity, and its transparency and validity in each model feature. Overall, SMART promotes fit-for-purpose modelling that is appropriate for the specific decision context and the constraints that come with it.
What prompted the need to create this tool?
In short, SMART was born out of the need to make decision models more accessible, more transparent, and more useful for those who need it most, especially when time and data are in short supply.
When we first started this work, our goal was to develop practical guidance for researchers who aren't health economics experts but still need to use decision-analytic models to inform important healthcare decisions.
We were thinking not only of scientists and innovators working on drug repurposing research in academic settings or SMES, but of anyone who, despite having limited time, data or technical expertise, still needs to assess the value of their healthcare innovation.
Why is this important within a healthcare innovation context?
There is currently no clear accessible guidance tailored to developing decision models in contexts such as designing an early-stage intervention, testing a repurposed drug, or working in a resource-constrained setting.
Decision-analytic models are powerful tools in health economics, used to compare healthcare interventions based on their potential cost-effectiveness. They support decisions at all stages of development, from research prioritization to pricing and reimbursement. But building these models isn’t straightforward.
As we looked through the existing literature and resources, we saw a growing gap in supporting decision models development in contexts where simplicity isn’t just a preference, but a necessity.
Think, for example, of precision mechanism-based drug repurposing, where hundreds of drug-indication pairs need to be screened for potential value, often without clinical or cost data. Or rare diseases and health system planning in low- and middle-income countries, where timelines are tight and data is sparse. In these scenarios, simple early-stage models can provide immense value, by highlighting unmet medical needs, estimating societal value and guiding early pricing discussions.
Who are the end users of SMART?
We believe SMART will be valuable to a wide range of users — from health economic decision modellers all the way to regulatory bodies, health technology assessment agencies, peer reviewers, policymakers, clinicians, and even other healthcare decision makers.
Apart from enabling reviewers and users to better assess whether a model is suitable to inform healthcare decisions, we also see value in using SMART as a training and educational tool for non-experts in health economic decision modelling, including scientists, innovators and public health professionals.
Now that it’s been officially released, we’re excited to see how SMART will be applied in real-world settings to support more transparent, efficient and fit-for-purpose modelling.
How has it been received by the healthcare community so far? Have you had any feedback from peers who have used the tool yet?
Yes! One of my colleagues is already using SMART in her own modelling research paper. She’s conducting an early value assessment (EVA) to evaluate the potential cost-effectiveness of seven digital technologies in addition to standard care in the United Kingdom; she said she found SMART very helpful to ensure transparent reporting and justification of the simple modelling choices.
We are also currently applying SMART in a REPO4EU pilot study involving patients with thyroid cancer. We can already see the value of our tool in guiding the development of a health economic decision model within a context of limited data and urgent need for new therapeutic options, as seen in patients with anaplastic thyroid cancer and poorly differentiated thyroid cancer.
What was your role in the development of SMART? And who else was involved in the process?
I developed SMART from its initial concept through to design and implementation, with the continuous support and guidance from Manuela Joore and Sabine Grimm, both also from Maastricht UMC+. My primary responsibilities included identifying gaps in existing health economic modelling guidance, shaping the framework and content of the tool and conducting interviews and workshops to help guide the SMART development.
The whole process also benefited from the contributions of Mirre Scholte from Radboud UMC and others from the REPO4EU consortium, including Prof. Joe Loscalzo and Adam Raymakers from Harvard Medical School. Together we identified key features of health economic decision models based on reviewing key literature, established guidelines, and our collective expertise.
To facilitate consistent use of SMART, we also developed a glossary and applied the tool in an illustrative case for planning a health economic decision model for a repurposed drug for treatment-resistant hypertension.
While the core development was conducted at KEMTA (the Clinical Epidemiology & Medical Technology Assessment at Maastricht UMC+) we also obtained extensive feedback through in-depth interviews and expert workshops with health economic decision modellers and model users, which helped us massively in refining the tool and inform key development decisions.
How will SMART contribute towards the development of the REPO4EU Platform and the overall mission of the project?
With the support of the Egnosis team, SMART will be integrated into the REPO4EU Platform to make it directly accessible to researchers and innovators engaged in drug repurposing research.
With the help of SMART, alongside other essential HTA resources on the platform, we aim to empower these researchers to develop health economic decision models to assess the value of their innovations independently and efficiently. This, in turn, promotes a more transparent and systematic approach to health economic evaluation in mechanism-based drug repurposing and precision medicine development, ultimately supporting improved decision-making and resource allocation for developing new therapeutic options that address unmet patient needs.

The SMART tool
Can we make health economic decision models as simple as possible, but not simpler?
Sounds interesting?
Follow the link to access version 1.0 of the SMART tool
Drug Repurposing Next-Gen: Interview with Filipa Lopes (University of Porto)
Filipa Lopes is a specialist in Bioethics, with a background in Biology and Applied Microbiology. She’s currently pursuing her PhD as part of the Ethics team within REPO4EU, where she’s conducting rigorous ethical analysis to ensure all the activities carried by the partners in the project adhere to the highest ethical standards — from animal welfare to patient data protection. In this interview, Filipa shares her journey as a young bioethicist and reflects on the importance of conducting research responsibly.
The role of Bioethics in scientific research
Hi Filipa! Let’s start with the beginning of your scientific journey. What inspired you to pursue a career in science?
My journey started with a Bachelor's in Biology at the University of Porto, followed by a Master's in Applied Microbiology at the Catholic University of Portugal. It was during my master's thesis, where I explored the bioethics of genome editing, that I discovered my passion for bioethics. That interest led me to pursue further specialisation, including a post-graduate degree in animal welfare. Professionally, I worked in evidence-based veterinary medicine before joining REPO4EU as a research fellow focused on ethics. I've also taken courses in clinical and research ethics, which further shaped my perspective.
For those unfamiliar with the term, can you explain what bioethics is and why it's important?
Bioethics is, in short, the way to do science with rules and values. It provides researchers with principles and guidelines that help ensure their work is done responsibly. In the rush to publish or innovate, some might overlook ethical considerations, and that’s where bioethics steps in — to define boundaries, safeguard participants and protect the integrity of research.
And when it comes to powerful technologies like genome editing or AI, what are the key ethical concerns?
Technologies like CRISPR-Cas9 are revolutionary but also raise serious ethical questions. While they offer incredible opportunities, they can also be misused, even for things like bioterrorism. We must apply a precautionary principle — thinking through consequences, risks and societal implications before using such tools, especially in humans.
In the case of Artificial Intelligence, this is still a new world for many of us, and its ethical implications are vast. Issues like bias, transparency, and accountability need careful attention. We're still learning how to navigate this space, but our goal as bioethicists is to ensure that new tools are used responsibly.
You mentioned you did a post-graduate course in animal welfare. What attracted you about this area specifically?
Animal welfare is essential in clinical research. In Europe we have to follow very strict rules and adhere to the 3Rs: Replacement, Reduction and Refinement. This means we always strive to minimise animal use, improve their living conditions and replace them when alternatives exist. I’m very passionate about this subject because I do understand that, in some instances, it is necessary to use animals for research because it’s for a huge end — for example, for clinical trials for cancer — but we must do so respectfully and ethically, always.
Developing an ethics-by-design framework for REPO4EU
Let’s talk about your work in the REPO4EU project. What is your role within this European consortium?
I’m currently pursuing my PhD within the ethics team at REPO4EU. We take an ethics-by-design approach to create tools like ethics self-assessment templates for all work packages, helping researchers in the project identify and address ethical issues from the start. We also develop information sheets and consent forms tailored for clinical trials, organise training on research ethics and open science, and ensure compliance across sensitive topics such as data privacy, use of human tissue, animal welfare and the integration of AI.
You mentioned open science and research integrity, how do these two fit in?
Research integrity deals with values and norms that distinguish acceptable from unacceptable scientific behavior, while research ethics applies foundational principles to protect participants and ensure transparency. Open science, in turn, promotes inclusiveness and democratic access to knowledge. Together, they create a framework for ethical, responsible and impactful research.
Have you encountered any challenges in getting researchers to adhere to this ethical framework?
Thankfully, no. Most researchers involved in REPO4EU already had a strong understanding of the importance of ethics. The biggest challenge we face is staying updated with everything happening across such a large, multidisciplinary project; it takes time to connect with all work packages and ensure alignment, but it's essential for applying our ethics-by-design framework effectively.
And what has it been like working with such a diverse, transdisciplinary team?
It’s both exciting and complex. Each REPO4EU partner brings a different expertise and degree of knowledge across AI, clinical trials, legal frameworks… as bioethicists, we need to understand enough of each field to offer meaningful ethical guidance; this means constantly learning and adapting. But I really enjoy this aspect of my work, it's one of the most enriching parts of the project.
Advice for young scientists and future researchers
What’s one key message you’d like other researchers to take from our conversation?
Be a responsible researcher. Embrace integrity, curiosity, honesty and resilience — and above all, respect science and the people involved in it. It's not just about the results, but about how we get there. Ethics, values and norms should guide everything we do.
And, drawing from your own journey and experiences, what advice would you give to a young person thinking of pursuing a scientific career?
Follow what makes you happy. Science isn’t easy, especially in countries like Portugal where funding is limited, but if it’s your passion, go for it! Be aware of the challenges, but don’t let them stop you. Resilience and love for what you do will carry you forward.
REPO4EU: The Podcast
Our podcast brings listeners closer to the latest innovations, research and developments happening in drug repurposing across the globe. The first season, ‘Drug Repurposing Next-Gen’, spotlights the work of PhD researchers, post-docs and young investigators involved in REPO4EU, exploring their role in the project as well as their career journeys. New episodes will be released monthly. Stay tuned for the next one!
Drug Repurposing Next-Gen: Interview with Dr. Fernando Delgado-Chávez (University of Hamburg)
Dr. Fernando Delgado-Chávez is a bioinformatician and computational biologist with an expertise in generative AI, currently working as Junior Group Leader at University of Hamburg’s Institute for Computational Systems Biology (CoSyBio). In this interview he talks about his journey so far as a young and reputed scientist, how AI can truly become an ally for researchers, and how he’s using network medicine principles to build software systems for the REPO4EU platform.
Building a solid career in Bioinformatics
Hi Fernando! Let’s start at the beginning: what led you to pursue a career in science?
That’s always a tricky question, when you’re 18 no one really knows what they want to be doing for the next 40 years! But in my case, as a teenager, I was fascinated by biology. I was amazed by how cells store so much information in their DNA, this genetic “code” that makes up who we are. I’ve always been curious about how the body works at the microscopic level, and I knew I wanted to do something that could help people. I wasn’t suited to work in a hospital — I’m actually afraid of blood! — but I knew I could contribute through other areas.
You studied biotechnology and then specialised in bioinformatics and health biotechnology. For people unfamiliar with those fields, how would you explain what they involve?
Essentially, biology, medicine and computer science all go hand in hand now. Bioinformatics is about studying how cells work, not just piece by piece, but as a whole system and it involves lots of data processing; that’s where computer science comes in. We can now analyse huge datasets and understand complex diseases in ways that weren’t possible before. It’s definitely an exciting time to be doing this kind of work.
After your postgraduate studies, you decided to do a PhD instead of going into industry. What made you choose that path and what was the focus of your research?
What really drove me was my love of learning. A PhD is very different from previous studies, it requires a lot of self-guided discovery, digging deep into topics that interest you, and that autonomy really suited me.
My research was about understanding what goes wrong in our cells when complex diseases like cancer develop. I focused on analysing gene expression; essentially, which genes are being “switched on” or “off” in diseased versus healthy cells. It’s like working with massive spreadsheets of numbers that represent biological activity, and we used computational algorithms to identify disrupted mechanisms. The goal was to create a system for interpreting this data and finding new therapeutic targets.
You’re now based in Hamburg, working at the Institute for Computational Systems Biology. What does your current role look like?
I started in Hamburg as a postdoctoral researcher, continuing my work on algorithms and disease mechanisms. While working with the CoSyBio team, I’ve also discovered a new passion: generative AI. In my research, I now focus mostly on how tools like ChatGPT can make biomedical research more efficient. And as a Junior Group Leader, I also manage a small team of master’s and bachelor’s students, so I spend a lot of time mentoring, strategizing and collaborating. It’s a real mix of hands-on research and project management.
Generative AI is such a hot topic right now. What would you say to researchers who are skeptical or nervous about using these tools?
AI is transforming the way we work across so many industries, and it’s here to stay, but we need to learn how to make the most of it. In research, for example, there’s just too much information out there, hundreds of new papers are published every week and it would take a lifetime to catch up and read everything.
AI tools like DrugRepoChatter, which I spearheaded the development for, can help us sift through a high volume of scientific literature much faster, which in turn makes the whole research process more streamlined and efficient. Of course, responsible use is key. Just like we once had to learn how to use Google effectively, now we need proper training on how to use AI wisely.
Building the REPO4EU platform with a network medicine approach
Let’s talk about your involvement in REPO4EU. What’s your role in the project?
I’m part of Work Package 2, where we build the bioinformatics software that powers the REPO4EU platform. I work on designing user-friendly tools that help researchers analyse molecular data to identify the root causes of disease, using the principles of network medicine. But I also contribute beyond that. I see myself as a generalist, and I’m also passionate about science communication and user-centred design, which is something I bring to the table within the context of the project. I spend a lot of time talking to future users of the platform to make sure we’re building something truly useful.
One of the aspects I love the most about being part of this project is that I get to work with experts from many different disciplines: computer scientists, clinicians, legal experts, communication teams… It’s an amazing learning opportunity, and it reflects how science really works today — it’s never in isolation.
In a nutshell, what is the REPO4EU platform and who is it for?
The platform is still in development, but its goal is to streamline the entire drug repurposing process. First, it helps researchers analyse biomolecular data to identify what’s going wrong inside a patient’s cells. Then it helps find existing drugs that could potentially target those disruptions. From there, researchers can use the platform to plan and manage preclinical and even early-phase clinical trials, and it also provides guidance on patents and regulatory issues. It’s an all-in-one online hub for anyone working on drug repurposing.
Advice for young scientists and future researchers
You said earlier that you enjoy communicating about science and exploring creative ways of making research more accessible to others. Do you see science and creativity as areas that go hand in hand?
Definitely. Science is one of the most creative activities I can think of. We’re constantly thinking of new ideas, solving problems, and designing experiments. Creativity is essential to innovation, and I think there’s a huge overlap between artistic thinking and scientific thinking.
Actually, before diving into science, I worked as a wedding photographer and did a lot of social media content. I’ve always been passionate about visual storytelling, and I think that background really shaped my approach to science communication. I strongly believe that scientists should be more visible, we need to give science a face so people can see beyond the data and the numbers. Social media can be a great tool to bridge that gap, that’s why I’m very active on LinkedIn, because it’s a great way to share my work with wider audiences.
Let’s finish our conversation with a piece of advice for young people considering a career in science. What would you say to them?
Don’t be afraid of science. I wasn’t the best student in chemistry or maths, but I was passionate about biology and I stayed curious. Science teaches you how to think critically, how to solve problems, and how to collaborate. Even if you don’t end up working in science forever, the skills you gain will shape your thinking for life. If you’re curious and you love learning, then you’re probably already on the right path.
REPO4EU: The Podcast
Our podcast brings listeners closer to the latest innovations, research and developments happening in drug repurposing across the globe. The first season, ‘Drug Repurposing Next-Gen’, spotlights the work of PhD researchers, post-docs and young investigators involved in REPO4EU, exploring their role in the project as well as their career journeys. New episodes will be released monthly. Stay tuned for the next one!
Drug Repurposing Next-Gen: Interview with Johannes Kersting (Technical University of Munich)
Johannes Kersting is a bioinformatician and PhD student at DaiSyBio, the research group led by Prof. Markus List at the Technical University of Munich. In this interview he talks about his journey into bioinformatics, what motivates him as a researcher, and how he’s building a data-driven knowledge base for drug repurposing as part of his involvement in REPO4EU.
A clearly defined path towards Bioinformatics
Johannes, you’ve focused all your studies in Bioinformatics so far, including your degree, masters and now your doctorate. What first drew you to this field in particular?
What attracted me to bioinformatics was how it brings together so many scientific disciplines. I enjoyed science in school — especially problem-solving — but wasn’t sure which field to focus on. Bioinformatics offered a mix of biology, chemistry, mathematics, computer science, and even physics. That interdisciplinary nature really appealed to me.
And what’s your favourite part about working as a bioinformatician?
I’ve always liked programming, so the computer science side was a big plus. But what really stood out was that bioinformatics is still a relatively young field. It emerged because biology started producing so much data that people needed new tools to make sense of it all. Since it’s still evolving, there’s a lot of room to contribute and shape the direction of the field, and that’s very exciting to me.
After finishing your masters, you chose to do a PhD, instead of going into industry. What made you take the academic route?
Mainly the chance for personal growth. In academia, you're constantly learning new things and being challenged with new problems. In the last two years alone, I’ve grown so much, not just in terms of knowledge, but in how I think and approach problems. That continuous learning is a big motivator for me.
What’s the main focus of your PhD research?
I’m studying gene regulation in the context of complex diseases. These diseases are often influenced by changes in how genes are regulated, so understanding those mechanisms can help us better understand disease progression — and ultimately improve treatments, including drug repurposing strategies.
Building a knowledge base for drug repurposing
Let’s talk a bit about your role in REPO4EU. What’s your main contribution to the project?
I work on the REPO4EU knowledge base. Drug repurposing relies on huge amounts of data, from drug-target interactions to side effects and disease associations. This information exists, but it’s scattered across different databases; our goal is to bring all of that together into a unified, accessible knowledge graph. That way, researchers across the project — and eventually, the wider scientific community — can work with the data more effectively.
On a day-to-day basis, what does this work entail?
It requires a lot of programming! I spend most days integrating new data sources, writing and testing code, and making sure everything is up to date. We’re building on an existing resource called NeDRex and extending it for REPO4EU. It’s very hands-on, which I really enjoy.
REPO4EU brings together people from a wide range of backgrounds — clinicians, software developers, legal experts, data scientists. Have you seen any benefits to being part of a diverse consortium?
I believe having an interdisciplinary approach in a project like REPO4EU is absolutely essential. Drug repurposing spans so many areas of expertise that no one person could master them all. You need people from pharmacology, biology, bioinformatics, software engineering, law, and more. Collaborating with people from other disciplines not only helps me solve problems more effectively, it also helps me see things from a different perspective. There are many examples I can think about that reflect this!
Is there one example in particular that comes to mind?
Yes, a small but funny one! A student in our group built a web tool that visualises gene interactions. We used technical gene identifiers that are easier for computers to handle. But then we got feedback from biologists in the project who said, “These numbers don’t mean anything to us!” So we changed it to use more readable gene names. It’s a great reminder that even if biotechnologists have a biology background, we’re not the end users, and working with peers from different disciplines help us achieve the best route that works for all of us.
Before joining REPO4EU, were you familiar with drug repurposing as a field?
Not really! I had a general idea, but I learned most of what I know now through my PhD and being involved in REPO4EU. What keeps me interested is how relevant this field is becoming. Drug repurposing won’t just be a “nice to have”, it will be a necessity. And like bioinformatics, it’s a young and evolving space, there are still many open questions, which means there’s room for creativity and innovation.
A word of advice for young researchers
Being a researcher can be challenging sometimes. What keeps you motivated to do what you do?
Honestly, I just really enjoy solving problems, that’s what drives me. It could be any research area — if there’s a challenge, I’m in. But I also draw a lot of inspiration from my team. Discussions with my supervisor, my colleagues at DaiSyBio, and collaborators in REPO4EU really help me develop and refine ideas. Those conversations are often where abstract concepts start to feel real, and it also helps me feel supported in my journey.
To wrap up, what advice would you give to a young researcher just starting out?
I’m still early in my career myself, so I don’t think I’ve been doing this long enough to give wise advice! But, if I have to say something that could’ve helped me at the beginning of my research career, I’d say: don’t get intimidated by how much you don’t know. That feeling is completely normal. When I started my PhD, I realised I knew way less than I thought I did — but that’s part of the journey. It gets better with time, and you really do learn something new every day. You’ll never know everything, and that’s okay.
REPO4EU: The Podcast
Our podcast brings listeners closer to the latest innovations, research and developments happening in drug repurposing across the globe. The first season, ‘Drug Repurposing Next-Gen’, spotlights the work of PhD researchers, post-docs and young investigators involved in REPO4EU, exploring their role in the project as well as their career journeys. New episodes will be released monthly. Stay tuned for the next one!
Calling for papers! Submit your research to our brand-new Network and Systems Medicine journal
About Network and Systems Medicine
Network and Systems Medicine is an open access, peer-reviewed journal focused on interdisciplinary approaches to exploiting the power of big data by applying network science and systems thinking to medicine. Network and Systems Medicine publishes high quality basic science, translational, and clinical research in the form of original research articles, comprehensive review articles, mini-reviews, rapid communications, brief reports, technology reports, hypothesis articles, perspectives, and letters to the editor.
The journal coverage includes:
- Network science applied to medicine
- Mechanism-based disease definitions, diagnostics and therapies
- Network medicine and network pharmacology
- Multiscale modeling and medical simulation
- Multiscale medical data science and computing
- Virtual patient repositories and data analytics platforms
- Big data analytics in precision medicine
- Clinical validation of systems medicine approaches (including quali-quantitative methods)
- Multiscale approaches, e.g. translational and qualitative research and psycho-sociological variables
- Implementing systemic management in medicine in organizations and integrated healthcare networks
- Educational and training articles in systems medicine and network medicine methodologies for clinicians
Call for papers
Learn more about key topics covered and the editorial board behind the Network and Systems Medicine journal
Contribute to Network and Systems Medicine
Explore all the details at Drug Repurposing Central and submit your research today!
Looking for a Premier Publishing Portal for Drug Repurposing Research?
Created as a joint enterprise by REPO4EU and ScienceOpen, Drug Repurposing Central is the premier publishing portal for researchers specializing in drug repurposing, network medicine and precision healthcare. Our portal provides a comprehensive list of resources collating the latest research, and offers multiple publication channels, including preprints, journal articles, conference abstracts, reports, and books.
DrugRxiv, as a cornerstone within Drug Repurposing Central, enhances the portal by allowing researchers to share preliminary findings rapidly. This unique collection supports the drug repurposing community by providing a dedicated space for early results, fostering collaboration and innovation.
Researchers can actively participate in sharing and discussion within an open review environment, powered by ScienceOpen’s innovative discovery infrastructure. Developed under the Horizon Europe project, REPO4EU, which aspires to establish a European Platform for Drug Repurposing with global outreach, Drug Repurposing Central offers unparalleled access to publishing and reading resources at no cost. This portal is steadfast in its commitment to advancing Open Science, ensuring the utmost integrity of research outputs through consistent use of persistent identifiers and XML standards.
Drug Repurposing Next-Gen: Interview with Dr. Ana Casas (University Hospital Essen)
Dr. Ana Casas is Assistant Professor in Neurology at University Hospital Essen in Germany, where she also leads a research group focused on network pharmacology for neurovascular diseases. In this interview she shares insights into her academic journey, challenges as a young researcher, and her involvement in the REPO4EU project as work package leader.
An academic journey fuelled by a passion for science
Ana, your academic background is fascinating—it combines neurology, pharmacology, and biotechnology. What led you to pursue these fields?
Like many scientists, I was driven by curiosity. As a kid, I loved learning how the body works. I was fascinated by forensic science TV shows, which sparked my interest in biology. When the time came to choose a degree, biotechnology was still new in Spain, but I decided to go for it. Later, during my studies, I became particularly interested in pharmacology—understanding how drugs work and how we can improve them. That led me to specialize in neuropharmacology during my master's.
For those unfamiliar with the term, can you briefly explain what neuropharmacology is?
Of course! Neuropharmacology focuses on the development and understanding of drugs that target neurological, psychiatric, and neurovascular disorders—such as stroke, schizophrenia, and Alzheimer’s. In my case, I specialized in neurovascular diseases and the mechanisms underlying conditions like brain ischemia.
What made you look for new opportunities outside of Spain?
It was quite unexpected! During my master’s, I worked on a neurovascular disease in a lab that collaborated with Professor Harald Schmidt at Maastricht University, who is now the REPO4EU coordinator. When I was finishing my master’s, he offered me a PhD position. Two days after submitting my thesis, I moved to the Netherlands and started my PhD research.
What was the focus of your PhD?
I continued researching neurovascular diseases, but at a more mechanistic level—trying to identify specific targets and potential drugs to treat brain ischemia. My goal was to validate new therapeutic approaches for stroke patients.
Many young researchers would agree that PhD journeys are full of opportunities but also challenges. What were some of the biggest obstacles you faced?
Science comes with constant challenges. You’re fighting the unknown, dealing with technical difficulties, and facing personal struggles. Moving abroad at 22, starting from scratch, handling bureaucracy in a foreign language—it was tough for me. But if there’s one thing a PhD teaches you, it’s resilience. You learn to cope with frustration, adapt, and always have a Plan B, C, or even D!
What kept you going?
Passion. Science isn’t just a job—it’s a vocation. You need to believe in your work, your field, and your team. That passion fuels you even in the toughest moments.
Joining REPO4EU and redefining the future of medicine
Let’s talk about REPO4EU. How did you get involved in this EU-funded initiative?
By the time REPO4EU started, I had moved to Germany, but I maintained my collaboration with Professor Schmidt. Given my expertise in preclinical research and animal studies, I was invited to join the project. It was a natural continuation of our work together.
What’s your role in the project?
REPO4EU is a large consortium covering everything from target identification to business development. My work focuses on preclinical validation—testing potential therapies in vitro and in vivo before they move to clinical trials. Essentially, we lay the foundation for later stages of drug development.
Interdisciplinary collaboration is key in such projects. How has working with experts from different fields influenced your approach?
It’s been a game-changer. Scientists often get stuck in their niche, but projects like REPO4EU push us beyond that. I work with clinicians, bioinformaticians, patenting experts, and business strategists—all with the shared goal of improving medicine. It’s a unique and enriching experience.
Drug repurposing has been gaining a lot of traction in the last few years. How does it feel to be part of this movement?
It’s incredibly exciting! Our work has the potential to redefine medicine—not just by accelerating drug development but by making treatments more accessible and efficient. Being part of something that can impact healthcare at a global level is truly rewarding.
A word of advice for young researchers
To wrap up, drawing from your own experiences, what advice would you give to young scientists considering a research career?
Fight for your passion. Science isn’t easy, and the path won’t be straightforward, but if it’s what you love, it will be worth it. Don’t let anyone tell you what you can or can’t do—believe in yourself and push forward.
REPO4EU: The Podcast
Our podcast brings listeners closer to the latest innovations, research and developments happening in drug repurposing across the globe. The first season, ‘Drug Repurposing Next-Gen’, spotlights the work of PhD researchers, post-docs and young investigators involved in REPO4EU, exploring their role in the project as well as their career journeys. New episodes will be released monthly. Stay tuned for the next one!
REPO4EU presents DrugRepoChatter, an AI timesaver tool for drug repurposing researchers
Sifting through the vast amount of scientific literature can be overwhelming, especially in fast-evolving fields like drug repurposing, where the sheer volume of publications, databases and tools available can pose a significant challenge for researchers trying to stay up to date with all the latest releases.
In a bid to tackle this problem, experts from the REPO4EU Consortium have joined forces to develop DrugRepoChatter, an AI-powered tool that helps researchers navigate through the relevant literature for mechanism-based drug repurposing in a faster and more efficient way.
Dr. Fernando M. Delgado Chaves, Research Group Leader at University of Hamburg’s Institute for Computational Systems Biology (CoSyBio), spearheaded the development of the tool, working alongside REPO4EU partners from other institutions to bring it to fruition. Drawing from his expertise in bioinformatics and generative AI, he explains in this interview his approach to the design and the methodology he adopted to create DrugRepoChatter, offering an up-close view on how it works and its capabilities.
Hi Fernando! Congratulations for the successful launch of DrugRepoChatter.
What prompted the need to create this tool?
Honestly, it's the sheer explosion of scientific papers out there! Keeping up, especially in a field that's moving as fast as drug repurposing, is just… insane. As researchers ourselves, we were constantly drowning in papers, trying to find the right tools, methods, and insights. It felt like there was so much great knowledge, but it was all scattered and hard to access efficiently.
That's the real problem DrugRepoChatter solves. We wanted to build something that acts like your personal expert assistant, helping you cut through the noise and find the crucial information fast. Think of it as taking all that scattered knowledge and putting it into one place, making it super easy to get to what you need. No more endless PDF wading! You ask a question, and boom, you get answers grounded in solid, curated literature.
How do you envision researchers using it? Can you think of a specific scenario of when they would be accessing it?
Picture this: you're a researcher, maybe a bioinformatician or data analyst, and you're knee-deep in a drug repurposing project. You've got this awesome dataset, and you're wondering, "What computational tools are actually good for this kind of data?" Instead of spending days Googling and digging through papers, you just hop onto DrugRepoChatter and ask, "What are the best AI tools for analyzing transcriptomics data in drug repurposing?" Within seconds, the chatbot gives you expert-selected articles that break down the best methods, compare them, and even link you to the databases you need. It's like getting instant, expert advice.
Or, imagine you're a clinician or biomedical researcher. You've got unique patient data and you're trying to figure out the right analysis techniques. Instead of getting lost in literature reviews, you ask DrugRepoChatter,“Which databases can I use to find validated clinical biomarker discovery in neurodegenerative diseases?” And bam! You get curated insights, pointing you to the best approaches backed by solid research. It’s purely about making research faster and smarter.
What degree of knowledge do researchers need to have about AI tools? Do they need prior experience using other similar platforms?
Zero, nada! Honestly, we made DrugRepoChatter super simple on purpose. You absolutely do not need to be an AI guru or have used chatbots before. If you can type a question, then you can use DrugRepoChatter – it’s as simple as that. Whether you're a seasoned pro or just starting out, we wanted to make sure this tool is accessible to everyone.
The database contains 285 open-access articles, which have been selected by experts from the REPO4EU.
Who was involved, and what was the selection criteria?
It has been a joint effort, with many colleagues from across the whole REPO4EU Consortium involved.
We had experts from all over Europe – University of Hamburg (UHAM), Technical University of Munich (TUM), GeneSurge, STALICLA, University of Vienna (UNIVIE), Brigham and Women’s Hospital (BWH), Maastricht University (UM), even Concentris! It was a big group effort.
And the selection criteria were pretty rigorous. We used a structured annotation process in Paperpile, where 13 of us from six different institutions collaboratively labeled publications. We categorized reviews by focus – clinical, computational, tools. Databases had to be usable, either downloadable or via an Application Programming Interface (API). Methods and tools were classified by strategy, technique, and usability – code, package, graphical user interface, API.
Basically, we wanted things that were actually useful for REPO4EU’s work, especially our tasks in WP2 on real-world data-driven AI in drug repurposing. And of course, they had to be either highly cited – 30+ PubMed citations – or very recent, published in the last 5 years.
I want to take this opportunity to mention some of the key people involved in the curation: Markus List, Quirin Manz, Judith Bernett and Johannes Kersting from TUM; Michael Hartung, Andreas Maier, Olga Tsoy and myself from UHAM; Emre Guney, Montserrat Puiggròs and Francesco Sirci at STALICLA; Julia Guthrie from UNIVIE; Ruisheng Wang at BWH; Robert Löwe from GeneSurge; Hermann Mucke from HMPC; and Harald Schmidt from UM.
As a bioinformatician, what was your role in this whole process?
I spearheaded the development, really making sure DrugRepoChatter brought together cutting-edge AI with a user-friendly design for researchers from all sorts of backgrounds. I’m personally fascinated by large language models (LLMs), and I quickly realized they were a game-changer for how we access scientific info. Think about the time we’re saving researchers by using AI!
I worked closely with computational biologists, clinicians, data scientists within REPO4EU. Collaborators like Lisa Marie Spindler, Farzaneh Firoozbakht and Andreas Maier were key in building the backend, while our clinical partners helped us make sure it was really useful from a translational perspective. It was truly interdisciplinary, which is what makes this project so special.
Now that DrugRepoChatter has gone live, how has it been received by the research community so far?
Have you had any feedback from researchers who have used it?
We know many researchers have accessed it already and are actively using it, which has been really encouraging! The response is mostly about how much time DrugRepoChatter saves on literature searches – that's a big win. This feedback is gold for us because it helps us keep improving the tool, making sure it really meets the needs of the community. We’re still refining it based on what users are telling us, but yeah, overall the initial response has been great!
You mentioned earlier you wanted to make this tool accessible to everyone.
Why was this an important element in the conception of DrugRepoChatter?
We wanted to go a step further than just open access. We aimed to make this knowledge even more accessible by building tools that make it easy to find and use. And let's be clear: DrugRepoChatter, and tools like it, are completely dependent on open access science. Imagine trying to build this chatbot with articles locked behind paywalls – it's impossible! The chatbot needs to read the articles, to understand them, to answer questions based on them. That's why open science isn't just important for researchers; it's fundamental to creating the very technology that can democratize scientific knowledge and accelerate progress.
DrugRepoChatter
Discover our Drug Repurposing Chatting Expert and start a conversation
Want to learn more?
Read the research article 'DrugRepoChatter: A Drug Repurposing Expert Chatbot Curated by the REPO4EU Consortium’ on ScienceOpen









